Intended user:
The device should be used only by physicians having adequate training
Contraindications:
1. Biopaper should not be used in patients with a known allergy to the ingredients of the product.
2. Patients allergic to shellfish should not use the product.
3. Biopaper should not be used to control hemorrhage from large arteries.
4. Biopaper should not be used for controlling post-partum bleeding or menorrhagia.
Limitations of use:
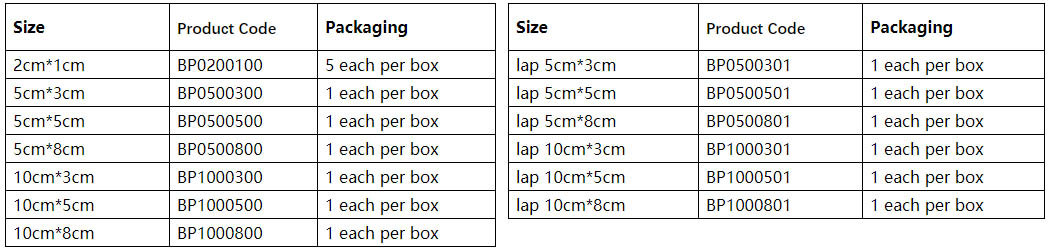
The maximum amount of usage for each person is 360 cm2 (Equivalent to 9 pieces of Biopaper with the size of 5cm×8cm).
Product lifetime:
According to experiment in animals, the Bio-paper could be degraded completely within 12 weeks after application.
Warnings:
1. Biopaper is provided sterile and are intended for single use. Do not resterilize. Reprocessing or re-sterilization may compromise the device integrity and could lead to patient injury, illness, or death. Reuse, even after re-sterilization, may create a risk of contamination and lead to patient infection or the transmission of infectious disease(s).
2. Biopaper should not be re-sterilized.
3. Use the device before the expiration date.
4. Never leave the plastic back holder and packaging materials inside the patient’s body.
5. Biopaper is not intended as a substitute for careful surgery and the proper use of sutures and ligatures.
6. Biopaper must be kept dry prior to application
7. The surgical field, especially desired site of application, should be cleaned to remove excess fluid before the application of the device.
Precautions:
1. Biopaper can be cut, folded, curled and covered to fit the wounds based on surgical needs.
2. If the placement position need to be adjusted, please move the Biopaper within 20 seconds, or the Biopaper becomes very sticky and not easy to be moved.
3. Foreign body reactions may occur with Biopaper, as with any implanted material.
4. Biopaper should be handled gently with dry instruments and/or gloves, and avoid contact with tissue surfaces until directly at the site of application, especially during the endoscopic process.
5. In the event of re-bleeding, apply one more Biopaper to the bleeding site directly.
6. Up to now, no hazards caused by interference between Biopaper and other equipment likely to be used in the course of other clinical procedures or medical treatments have been found.
7. It’s no hazards to use Biopaper in MR environment and there is no effect of Biopaper on the MR image artifacts.